Bradford Assay: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
The Bradford assay should be used to quantify total protein within a sample relative to a standard curve. This is important when running proteins on SDS-PAGE/Western blot, where each lane must contain an identical amount of total protein. This way, the intensity of the bands can be compared between different lanes, as standardizing the amount of protein loaded per lane will account for any variation in cell count from which the proteins were derived. | The Bradford assay should be used to quantify total protein within a sample relative to a standard curve. This is important when running proteins on SDS-PAGE/Western blot, where each lane must contain an identical amount of total protein. This way, the intensity of the bands can be compared between different lanes, as standardizing the amount of protein loaded per lane will account for any variation in cell count from which the proteins were derived. | ||
'''Protocol''' | |||
'''Protocol''' | '''Protocol''' | ||
| Line 14: | Line 15: | ||
# Using a 0.2 μm filter and 15 mL syringe, filter approximately 4 mL of Bio-Rad Protein Assay Dye Reagent Concentrate (Bradford Dye) into a 5 mL Eppendorf tube or 15 mL falcon tube. Let it warm to room temperature. | # Using a 0.2 μm filter and 15 mL syringe, filter approximately 4 mL of Bio-Rad Protein Assay Dye Reagent Concentrate (Bradford Dye) into a 5 mL Eppendorf tube or 15 mL falcon tube. Let it warm to room temperature. | ||
# Collect lysate or supernatant | # Collect lysate or supernatant from the cells. Keep on ice. | ||
# In separate 1.5 mL tubes, dilute the sample using ddH2O (or whatever buffer/media is appropriate) into 1:2 and 1:10 dilutions into a total of 600 μL. ('''NOTE:''' Do not use the 1:2 diluted sample to prepare the 1:10 tube. Use the original, undiluted sample to prepare the 1:10 dilution) | # In separate 1.5 mL tubes, dilute the sample using ddH2O (or whatever buffer/media is appropriate) into 1:2 and 1:10 dilutions into a total of 600 μL. ('''NOTE:''' Do not use the 1:2 diluted sample to prepare the 1:10 tube. Use the original, undiluted sample to prepare the 1:10 dilution) | ||
# Prepare a 1 mg/mL stock solution of BSA by dissolving BSA in ddH2O. Prepare a dilution series using ddH2O (or whatever buffer/media is appropriate) in 1.5 mL tubes: 1 mg/mL, 0.8 mg/mL, 0.6 mg/mL, 0.4 mg/mL, and 0.2 mg/mL. | # Prepare a 1 mg/mL stock solution of BSA by dissolving BSA in ddH2O. Prepare a dilution series using ddH2O (or whatever buffer/media is appropriate) in 1.5 mL tubes: 1 mg/mL, 0.8 mg/mL, 0.6 mg/mL, 0.4 mg/mL, and 0.2 mg/mL. | ||
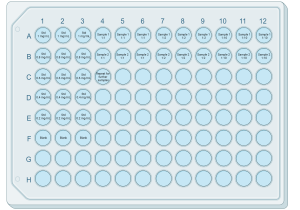
# See '''Figure 1''' for a general plate setup. Using the reverse pipetting technique, pipet 160 μL of blank, standard, and samples into each appropriate well. Avoid creating air bubbles. | # See '''Figure 1''' for a general plate setup. Using the reverse pipetting technique, pipet 160 μL of blank, standard, and samples into each appropriate well. Avoid creating air bubbles. | ||
# Spin down the plate at 300 rpm for 1 min to remove bubbles and any liquid on the side of the well. | # Spin down the plate at 300 rpm for 1 min to remove bubbles and any liquid on the side of the well. | ||
# | # Pour 4 mL of filtered Bradford Dye into a multichannel pipette reservoir. Using a multichannel pipette, pipet 40 μL of dye into each well. If a multichannel pipette is not available, '''''quickly''''' pipet 40 μL of dye into each well using a P200 pipette. | ||
# Incubate for at least 5 minutes at room temperature. Do not exceed 1 hr. | # Incubate for at least 5 minutes at room temperature. Do not exceed 1 hr. | ||
# Take off the lid of the plate, and place it into a plate reader/spectrometer. | # Take off the lid of the plate, and place it into a plate reader/spectrometer. | ||
Revision as of 19:29, 18 January 2022
Introduction
The Bradford assay should be used to quantify total protein within a sample relative to a standard curve. This is important when running proteins on SDS-PAGE/Western blot, where each lane must contain an identical amount of total protein. This way, the intensity of the bands can be compared between different lanes, as standardizing the amount of protein loaded per lane will account for any variation in cell count from which the proteins were derived. Protocol
Protocol
Some things to know:
- This protocol assumes the use of bovine serum albumin (BSA) for the standard curve, which has a linear range of 200 μg/mL to 1000 μg/mL within Bio-Rad's protein assay dye.
- If your protein is within a supernatant containing an acid indicator, such as phenol red, this may interfere with the assay. If your protein is within a solution containing phenol red, then ensure that all samples, standards, and blanks are diluted with that media (e.g. Serum free DMEM).
- Each tube will have extra volume to ensure you have enough for the last replicate and to avoid air bubbles.
- Reverse pipetting technique is highly recommended to avoid air bubbles and to ensure accuracy.
For a 96 well plate
- Using a 0.2 μm filter and 15 mL syringe, filter approximately 4 mL of Bio-Rad Protein Assay Dye Reagent Concentrate (Bradford Dye) into a 5 mL Eppendorf tube or 15 mL falcon tube. Let it warm to room temperature.
- Collect lysate or supernatant from the cells. Keep on ice.
- In separate 1.5 mL tubes, dilute the sample using ddH2O (or whatever buffer/media is appropriate) into 1:2 and 1:10 dilutions into a total of 600 μL. (NOTE: Do not use the 1:2 diluted sample to prepare the 1:10 tube. Use the original, undiluted sample to prepare the 1:10 dilution)
- Prepare a 1 mg/mL stock solution of BSA by dissolving BSA in ddH2O. Prepare a dilution series using ddH2O (or whatever buffer/media is appropriate) in 1.5 mL tubes: 1 mg/mL, 0.8 mg/mL, 0.6 mg/mL, 0.4 mg/mL, and 0.2 mg/mL.
- See Figure 1 for a general plate setup. Using the reverse pipetting technique, pipet 160 μL of blank, standard, and samples into each appropriate well. Avoid creating air bubbles.
- Spin down the plate at 300 rpm for 1 min to remove bubbles and any liquid on the side of the well.
- Pour 4 mL of filtered Bradford Dye into a multichannel pipette reservoir. Using a multichannel pipette, pipet 40 μL of dye into each well. If a multichannel pipette is not available, quickly pipet 40 μL of dye into each well using a P200 pipette.
- Incubate for at least 5 minutes at room temperature. Do not exceed 1 hr.
- Take off the lid of the plate, and place it into a plate reader/spectrometer.
- Measure absorbance at 595 nm.
Analysis using the standard curve
- Export results from spectrometer to Excel.
- Subtract the average absorbance of each well by the average absorbance of the blank.
- Plot the known standard concentrations (X-axis; 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) with the average absorbance at each concentration (Y-axis). Add a line of best fit and display the equation with R2 value. Ideally, the R2 value should be ≥ 0.99. R2 < 0.94 curves should not be used.
- Calculate the average absorbance for the samples of interest by using the equation of the line graph y = mx+b (Absorbance = Slope*Concentration + b) and solve for x.
- If the calculated concentration falls within the linear range of the standard curve (0.2 - 1.0 mg/mL), then it is accurate. If necessary, determine the concentration of the undiluted sample by multiplying by the dilution factor.